Services
Risk Management
BioTop Medical has extensive experience in medical devices risk management according to EN ISO 14971 and uses dedicated tools which support systematic analysis, evaluation and process compliance.
Risk management is a multidisciplinary exercise. It requires knowledge of the product design characteristics, manufacturing process, intended use, claims, mode of action as well as a deep understanding of the application of standards EN ISO 14971 (and underlying risk analysis techniques) and EN IEC 62366-1 which provides the rules for the risk management process including usability risk management.
BioTop Medical delivers the expertise in risk management of medical devices, ensuring compliance with the requirements of the regulations and standards, throughout the device life-cycle.
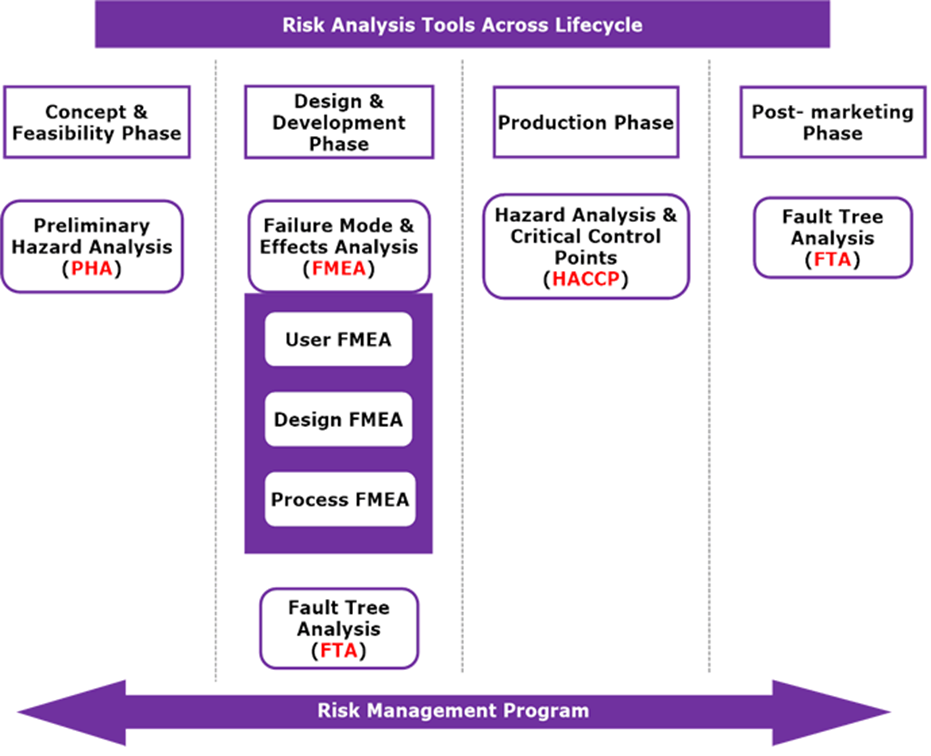
Since the risk management is supposed to be updated throughout the design and development stages, the traceability throughout these processes needs to be ensured. This starts with establishing risks to product (usability) requirements, specifications and risk control measures, leading to the verification and or validation of those measures and the evaluation of the residual risk. We excel in coordinating risk management processes and in writing and organizing the resulting documentation in a way that ensures traceability.

