Services
Clinical / Performance Evaluation
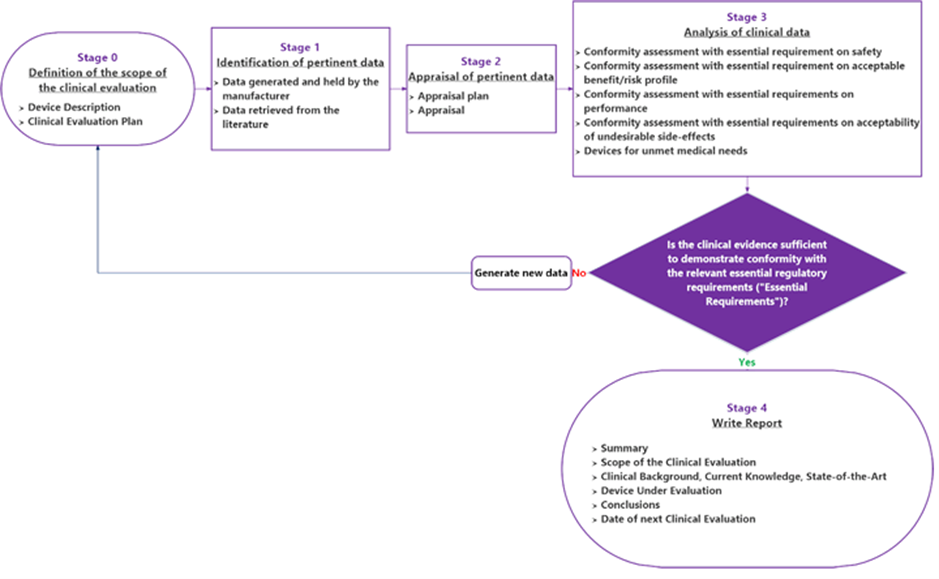
BioTop Medical has extensive experience in clinical / performance evaluation. We follow the requirements of the MDR / IVDR. We write the plan, objectively analyze the data sets, and write the conclusions. We deliver the expertise in the evaluation methodology, ensuring compliance with the requirements of the regulations.
Clients often ask: “How are you able to perform a literature search without being an expert on the subject of a specific medical device?”
The answer is the following: the first step is the client training us in the medical application of the device, including principles of operation, mode of action, known alternative technologies. With this information we are able to draft the literature search protocol which the client reviews and approves before we can start the search. During the search of the literature, our knowledge of the device application, its technology and the alternative technologies, keeps increasing. Only after this initial literature search (to define the state-of-the-art in the field of the device application) we will engage in the clinical or performance evaluation. In this way we are able to combine our experience in performing these evaluations with our client’s knowledge about the specific medical device.
Our (medical) scientific background, our experience with a large diversity of medical devices and our expertise in the evaluation method, enables us to quickly acquire the specific knowledge we need to perform the evaluation.
The clinical or performance evaluation is the assessment and analysis of data sets pertaining to a medical device to demonstrate compliance with a number of MDR / IVDR General Safety and Performance Requirements (GSPR) related to the device achieving of the claimed intended purpose, performance efficacy, benefits and safety, which require clinical data.
Clinical data can be obtained from one or more sources (e.g. literature, pre-clinical, clinical data) and it must be performed according to a defined and sound methodology as described by Article 61 and Annex XIV of the MDR / Article 56 and Annex XIII of the IVDR. In addition, the evaluation must be unbiased: thorough and objective, considering both favorable and unfavorable data.

